ThecaFlex DRx™ System

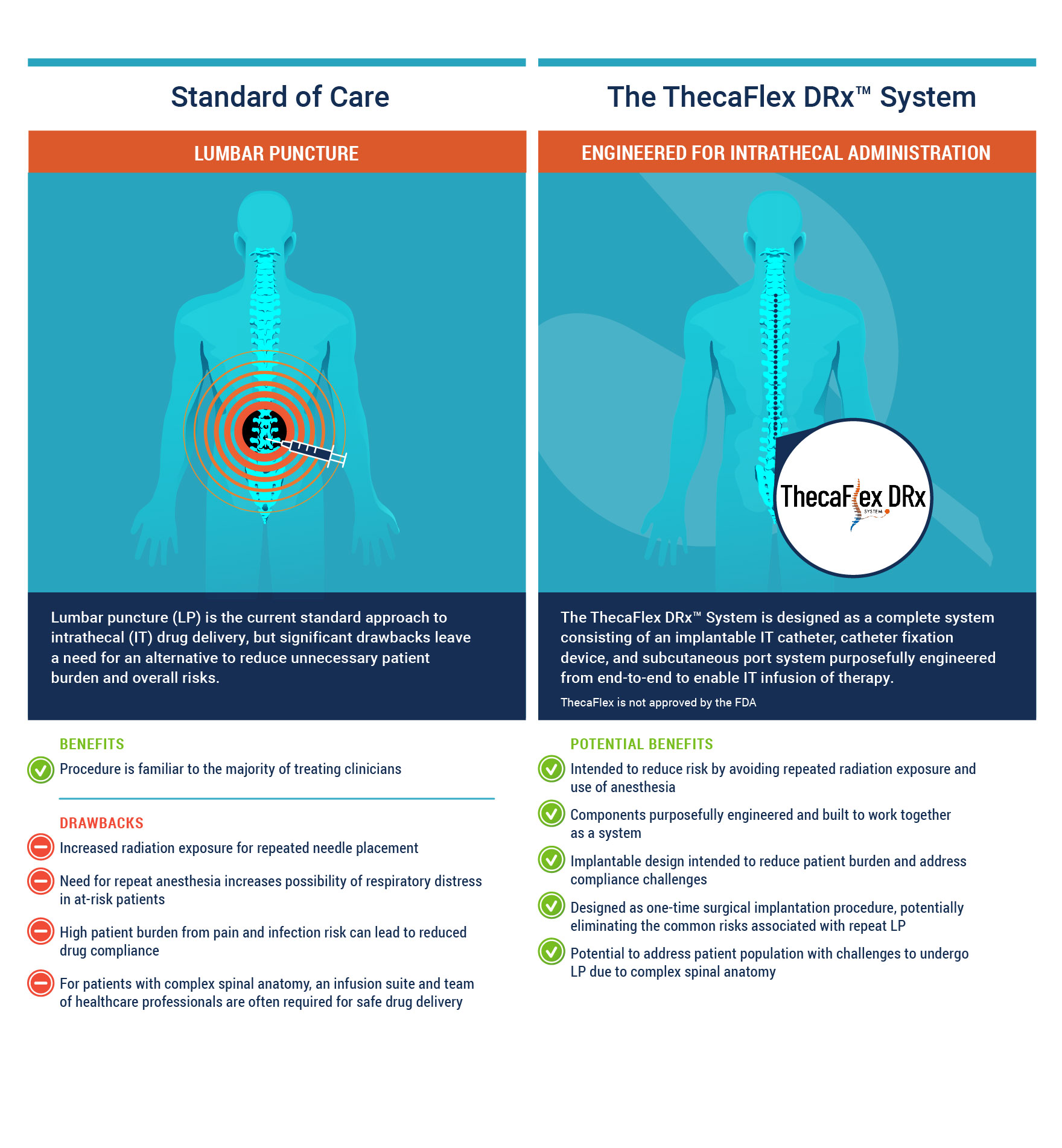
The ThecaFlex DRx™ System, a technology within Alcyone’s Falcon™ Delivery Platform, is an implantable intrathecal (IT) catheter, catheter fixation device, and subcutaneous port system designed to provide access to the cerebrospinal fluid (CSF) for the infusion of therapies by IT bolus administration.
Lumbar puncture (LP), commonly known as a spinal tap, is the current standard of care approach to delivering therapeutics into the CSF. During LP, a needle is inserted into the space between two vertebrae and into the CSF contained in the IT space. Emerging alternatives to LP include the implantation of off-label port-and-catheter systems that circumvent the discomfort and risks of repeat LPs, which frequently require anesthesia and radiation for safe delivery, and to provide an option to those who are not good candidates for repeat LP.
In response to the clear and growing unmet need for a better method of repeat IT drug delivery, the ThecaFlex DRx™ System is designed to be a completely implantable port-and-catheter system for direct IT access to the CSF. Currently there are no FDA approved implantable subcutaneous port and IT catheter systems indicated to provide long-term IT access, which could be used for bolus IT administration of approved drugs as well as for CSF aspiration. ThecaFlex DRx™ System enables bolus IT administration of therapeutics following a one-time surgical implantation procedure, eliminating the risks associated with repeat LP and the technical challenges of other port-and-catheter systems that are not designed or tested for IT delivery.
Patients may benefit from the use of the ThecaFlex DRx™ System as it facilitates a potentially safer treatment alternative for those with challenging anatomy and for those who would require multiple anesthesia and radiation exposures for repeat LPs.
Potential risks associated with an implantable system include surgical risks and device-related issues. The ThecaFlex DRx™ System is undergoing extensive testing including clinical trials to mitigate these risks. The key risks of this System includes infection, CSF leak, and device malfunctions such as leakage, catheter/system occlusion, and disconnection.
The ThecaFlex DRx™System has received CE Mark Approval in Europe and Breakthrough Device Designation from the Federal Drug Administration (FDA).
ThecaFlex is not approved by the FDA.
Alcyone is currently enrolling in an FDA-approved IDE clinical trial (NCT05866419) to assess the safety and performance of the ThecaFlex DRx™ System in subjects with Spinal Muscular Atrophy (SMA). The study is enrolling patients aged 3 and above who are receiving the FDA-approved, intrathecally delivered antisense oligonucleotide (ASO), nusinersen. The first use of the ThecaFlex DRx™ System occurred in 2023, and recruitment efforts for the clinical trial are ongoing.